Explain Briefly the Difference Between Atom and Molecule
And a compound is a type of molecule in which the types of atoms forming the molecule are different from each other. Structural Formula A structural formula tells us which atoms are in a molecule.
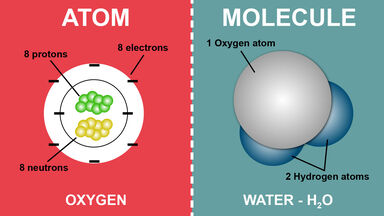
Basic Difference Between An Atom And A Molecule
An atom is composed of a nucleus made up of protons and neutrons and electrons orbiting around it.
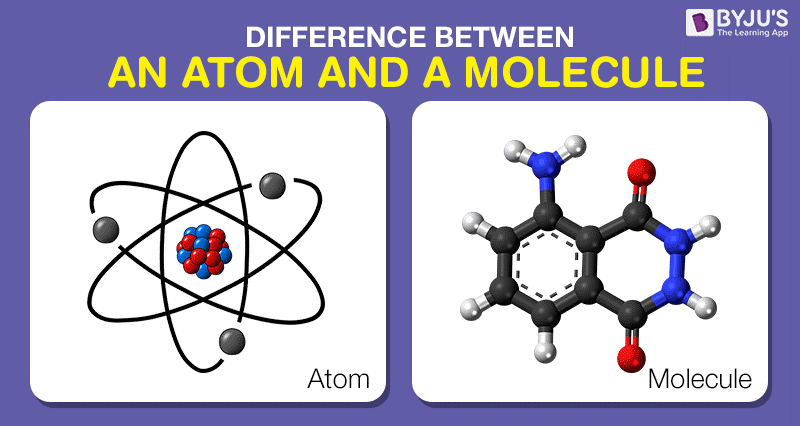
. The difference between atom and molecule can be drawn clearly on the following grounds. Explain the difference between electron pair geometry and molecular geometry. Thus we must learn the difference between atom and molecule for a better understanding.
The term covalent molecular is used to explain molecules that are formed by covalent bonding. There are different types of structural formula. Suppose there is a bond between.
In this article the difference between a molecule and compound in tabular form is given to help the students understand this topic better. Electron geometry includes the lone electron pairs present in a molecule. Two or more elements bonded together through ionic attraction.
The key difference between atomic spectroscopy and molecular spectroscopy is that the atomic spectroscopy refers to the study of the electromagnetic radiation absorbed and emitted by atoms whereas the molecular spectroscopy refers to the study of the. It is not possible to breakdown the atom further retaining the properties of the element. 5 Does O2 represent an atom or a.
Of carbon hydrogen and oxygen. Answer 1 of 3. On the other hand.
A structural formula uses lines to show the bonds between the atoms. And so theyre different when a molecule contains lone pairs. O2 is a molecule of oxygen.
2 Why is a molecule of O2 more stable than 2 separate atoms of oxygen. Briefly explain the difference in molecular chemistry between silicone polymers and other polymeric materials. Updated on December 10 2019.
An atom is the smallest component of an element whereas a molecule is made up of two or more atoms. This is the main difference between covalent molecular and covalent network. It also shows us how they connect to each other.
A covalent network is a compound composed of a continuous network throughout the material in which the atoms are bonded to each other via covalent bonds. You can also have molecules of a single atom bonded together like two oxygen atoms. A condensed structural formula omits most or all the bonds.
Na and Cl are atoms of socium and chlorine. The least complicated structural formula tells. There are different theories developed to determine the electronic and orbital structures of molecules.
Molecular geometry can be determined by the number of bonds that a particular molecule has. A molecule consists of two or more atoms. The atoms within a compound also must be different from eachother whereas a molecule can consist of only one element.
Electronegativity is the tendency of an atom to attract the shared pair of electrons in a covalent bond towards itself. A molecule of water is a compound as it has two atoms of Hydrogen and one atom of Oxygen. Difference Between Atomic Spectroscopy and Molecular Spectroscopy.
For example the atoms of element gold cannot be broken down. So while an atom is its own separate entity a molecule is what you get when those atoms bond together. Difference between molecule and compound is one of the most commonly discussed topics in the chemistry subject.
A molecule is a group of two or more similar or dissimilar atoms that are held together by chemical bonds. There are mostly covalent bonds between the atoms of the molecules. The main difference between electron geometry and molecular geometry is that electron geometry is found by taking both lone electron pairs and bonds in a molecule whereas.
State the bond angle found in an ammonia molecule. Unlike elements molecules can be made from the same or different elements. Explain why the bond angle in an amide ion is smaller than that in an ammonia molecule.
Draw the shape of an ammonia molecule and that of an amide ion NH2In each case show any lone pairs of electrons. All elements have certain electronegativity values based on Pauling scale. Answer Silicon polymers are constructed from chains of silicone and oxygen whereas other polymers mostly made from chains of carbon.
The key difference between molecular orbital theory and hybridization theory is that molecular orbital theory describes the formation of bonding and anti-bonding orbitals whereas hybridization theory describes the formation of hybrid orbitals. The key to a molecule is that two or more atoms are bonded together. Atom is defined as the smallest unit of an element which may or may not exists independently.
And this is because lone pairs uh exert a greater repulsion than bonding pairs. What is the difference between Atom and Molecule. So if we look at an atom like say methane ch four this contains no lone pairs and so the molecular geometry in the electron geometry are the same.
C H O are atoms. Barring Hydrogen and Helium the outermost atomic orbits of all other elements has a. Cl2 is a molecule of chlorine.
O is an atom of oxygen. If three oxygen atoms bond together you get. Difference Between Atom and Molecule An atom is smallest particle in an element that has the properties of the element.
4 What is the difference between atom and molecule. October 5 2018 Posted by Madhu. Compounds contain two or more atoms and are held together by ionic bonds whereas molecules are held together by covalent bonds.
Do Lewis Dot Structures depict structures with ionic bonds covalent bonds both ionic and covalent bonds or neither. Its actually made of two hydrogen atoms and one oxygen atom. Cl is an atom of chlorine.
Atoms are not visible to the naked eye and are the basic building blocks. Atoms may or may not exist in the free state but molecules exist in the free state. 1 LP 2 LP.
Learn about our Editorial Process. 1 How is O2 different from O atom. DEFINE both which is more accurate and WHY.
For example water is a molecule made of hydrogen and oxygen. Atoms are the smallest particles in any element which also possesses the properties of the element. A molecule is made up of atoms bonded together.
Atoms forming compounds form ionic or covalent bonds. These might be the same elements such as two oxygen atoms bonded together O2 or it might be different atoms bonded together like water H2O. Electrons are actually placed in several orbits or shells around the nucleus.
A molecule is formed when two or more atoms of an element chemically join together. Thus we cannot see atoms with our naked eye. C12H22O11 is a molecule of table sugar.
Nonetheless they are the building blocks of each and every material. NaCl is a molecule of table salt. This comparison of molecule and compound can also help the students to know the examples of a.
3 Which is more stable at room temperature a O2 molecule or two separate O atoms which is more stable at room temperature a molecule or two separate atoms.

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube

Difference Between Atom And Molecule In Tabular Form

Difference Between Atoms And Molecules Atoms And Molecules Definition
Comments
Post a Comment